Classifying
Chemical Reactions
| Back | Home | Next |
Decomposition Reactions
When a single reactant breaks apart to form
General form
XY-->+
Example
H2CO3(aq) → H2O(l) + CO2(g)
Thermal Decomposition
Some substances will only decompose when heated to.
Example
--> +
Just 100g of NaN3 can produce litres of nitrogen gas in seconds. This is used in
Combination (Composition) Reactions
When two or more reactants combine to form
General Form
X + Y -->
Example
H2(g) + Cl2(g) → 2HCl(g)
Displacement Reactions
![]() Replacement Reactions Video Part A
Replacement Reactions Video Part A
![]() Replacment Reactions Video Part B
Replacment Reactions Video Part B
Precipitation Reactions
This occurs when two clear liquid solutions are added and a appears and falls to the bottom of the solution.
Eg Lead Nitrate (aq) and Potassium Iodide (aq) --> Potassium Nitrate (aq) + Lead Iodide (s)
 Precipitation Reactions and Solubility
Precipitation Reactions and Solubility

A precipitation occurs when a substance will not dissolve (usually in water) and it is called .
Sugar is where as Chalk is in water.
Solubility is realted to the ability of ionic compounds (eg NaCl – salt) to break apart into its eg Na+ + Cl- when dissolved in water.
 Ionic Compounds
Ionic Compounds
Substances made up of a lattice (or crystal) structure. The substances have a positive ion called a and a negative ion called an .
There are normally , and brightly coloured.
The cations are atoms (or compounds) that have lost and the anions are atoms (or compounds) that have gained .
- Some common cations that have lost 1 electron are
- , , , and
- Some common cations that have lost 2 electrons, , , and
- Some common cations that have lost 3 electrons are and .
- Some Common anions that have gained 1 electron are ,, and
- Some Common anions that have gained 2 electron are , ,and CO32-
- Some Common anions that have gained 3 electron are and .
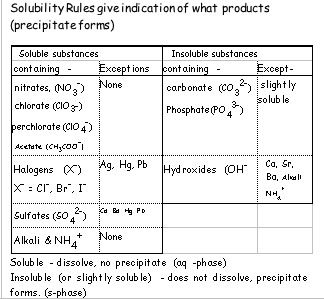
Predicting Precipitates
Scientists use a list of solubility rules to help them predict if something will dissolve or not
From the table predict which of the products will be a precipitate
KCl(aq) + AgNO3(aq) → KNO3() + AgCl()