Classifying
Chemical Reactions
| Back | Home | Next |
Decomposition Reactions
When a single reactant breaks apart to form
General form
XY-->+
Example
H2CO3(aq) → H2O(l) + CO2(g)
Thermal Decomposition
Some substances will only decompose when heated to.
Example
--> +
Just 100g of NaN3 can produce litres of nitrogen gas in seconds. This is used in
Combination (Composition) Reactions
When two or more reactants combine to form
General Form
X + Y -->
Example
H2(g) + Cl2(g) → 2HCl(g)
Displacement Reactions
![]() Replacement Reactions Video Part A
Replacement Reactions Video Part A
![]() Replacment Reactions Video Part B
Replacment Reactions Video Part B
Precipitation Reactions
This occurs when two clear liquid solutions are added and a appears and falls to the bottom of the solution.
Eg Lead Nitrate (aq) and Potassium Iodide (aq) --> Potassium Nitrate (aq) + Lead Iodide (s)
 Precipitation Reactions and Solubility
Precipitation Reactions and Solubility

A precipitation occurs when a substance will not dissolve (usually in water) and it is called .
Sugar is where as Chalk is in water.
Solubility is realted to the ability of ionic compounds (eg NaCl – salt) to break apart into its eg Na+ + Cl- when dissolved in water.
 Ionic Compounds
Ionic Compounds
Substances made up of a lattice (or crystal) structure. The substances have a positive ion called a and a negative ion called an .
There are normally , and brightly coloured.
The cations are atoms (or compounds) that have lost and the anions are atoms (or compounds) that have gained .
- Some common cations that have lost 1 electron are
- , , , and
- Some common cations that have lost 2 electrons, , , and
- Some common cations that have lost 3 electrons are and .
- Some Common anions that have gained 1 electron are ,, and
- Some Common anions that have gained 2 electron are , ,and CO32-
- Some Common anions that have gained 3 electron are and .
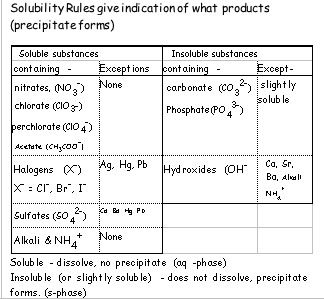
Predicting Precipitates
Scientists use a list of solubility rules to help them predict if something will dissolve or not
From the table predict which of the products will be a precipitate
KCl(aq) + AgNO3(aq) → KNO3() + AgCl()
Oxidation and Reduction Reactions
Not always evident when an reaction takes placeOxidation reactions
Usually occurring when is combined (joined) with another chemical. This can result in the reaction called
An example of combustion is
methane + oxygen → carbon dioxide + water
CH4(g) + 2O2(g) → CO2(g) + 2H2O(l)
 Combustion Video
Combustion Video
Reduction reactions
Usually occur when oxygen is from another substance. It is the of oxidation.
copper(II) oxide + carbon → copper + carbon dioxide
2CuO (s) + C (s) → 2Cu (s) + CO2 (g)
Here oxygen is removed from and added to .
Notice there is both a reduction reaction and an oxidation reaction occurring. This is called a .
Redox Reactions and electrons
Redox reactions do not always involve . They are many reaction that involve the transfer of from one substance to another.OILRIG – Oxidation Is L, Reduction Is G
Metal Displacement Reactions
These are replacement reactions which are simple redox reactions where the move from one metal to
Example
Cu2+(aq) + Zn(s) → Cu(s) + Zn2+(aq)
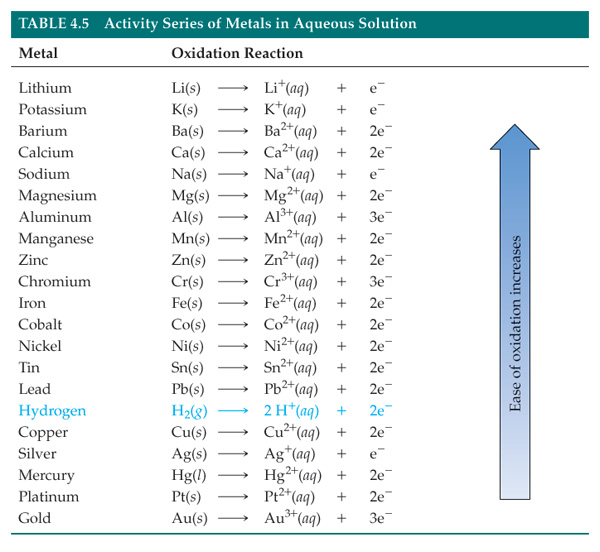
Activity Series
Replacement reactions do not work all the time. This is because some substances are reactive than others.The activity series (see table ) summarizes this.
Extraction of Metals
Metals are extracted from their . They bond to these substances because of their activity. Very non-reactive substances are usually found in form like and gold. Other very reactive metals are never found in their pure form and must be extracted.
Three groups are
- Native metals
- Electrolysis
The ones needing electrolysis are the most reactive.
Native metals
Metals are not very reactive like and . We find them in nature and they are separated from the surrounding sand and rocks by such things as crushing and .
Carbon Reduction
Naturally occurring metals found in . Some common ores are found in the table on page 156.Extraction is done by reduction where the ore is heated with a pure form of carbon (possibly coke) and oxygen is forced in.
The reduction of iron ore works like this in a step process
Step 1
carbon + oxygen gas → carbon monoxide gas
2C(s) + O2(g) → 2CO(g)
Step 2
Iron(III) oxide + carbon monoxide gas à iron + carbon dioxide gas
Fe2O3(s) + 3CO(g) → 2Fe(s) + 3CO2(g)
This whole process is known as
Electrolysis
Carbon reduction does not work with the very reactive metals like , orThe process whereby an electrical current is passed through the molten ore is called .
The electricity pushes the electrons onto the metal ions and thus then.
The process uses large amounts of electricity and is very expensive. Some common metals extracted this way are , and .
Sodium can be extracted from molten salt by electrolysis.
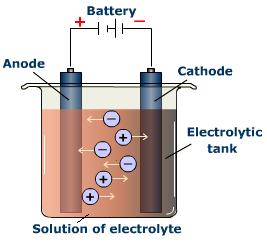
sodium + an → sodium
Na+(aq) + e– → Na(s)
And
chloride → chlorine gas +
2Cl–(aq) → Cl (g) + 2e–