Rates of
Chemical Reactions
| Back | Home |
Fast and Slow Chemical reactions
The speed at which chemicals react is called the
Quick chemical reactions might be and
Slow ones might be and .
Controlling the Rate of Reaction
Rates can be changed by a number of different variables
- Temperature
- of the reactants
- area
- Agitation ()
- Catalysts
Temperature
temperature will normally increase the rate of the reaction for two reasons
- the particles thus increasing collisions
- Particles have more to break bonds when they do collide.
Lowering the temperature can reduce the of reaction.
Concentration
Increasing the of the reactants increases the rate of reaction because there are more particles to .
Reducing the concentration can act to a process. Eg, reducing the amount of oxygen (air) can cause a fire to go slow of go out.
Agitation
Stirring adds energy to the molecules and so they more often.
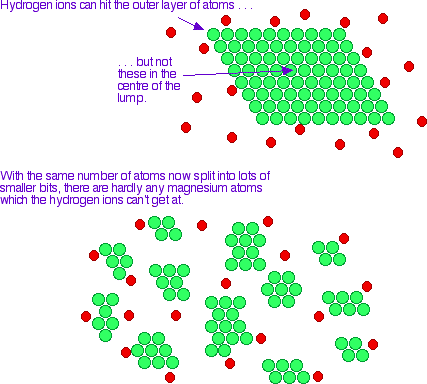 Surface Area of Reactants
Surface Area of Reactants

By increasing the amount of space a reaction can take place on causes a reaction to.
Catalyst
A is a chemical that speeds up a reaction without actually itself
They work by
- Reducing the amount of needed
- Make it easier for reactants to
Enzymes
are biological catalysts catalysts.
Enzymes make your body work right. You have enzymes in your body that do only job.
The enzyme in your mouth that breaks down sugar is